3 Spleen
3.1 Review
Diseases involving the spleen are relatively common in dogs, but less so in other species.
The spleen is a complex organ composed of two physical and functional spaces: the red and white pulp. It serves three main functions:
- Filtering out aged or altered RBCs and blood-borne particulate matter.
- Screening blood for pathogens.
- Storage of blood (RBCs and platelets in particular)
As with other organs, reviewing the normal structure and function of the spleen will help you understand the pathogenesis of the conditions described below. Chapter 13 of Pathologic Basis of Veterinary Disease provides a good overview, and can be accessed from the library.
A few key points are worth mentioning here, however.
The spleen is made up of red and white pulp.
- The white pulp is composed of the periarteriolar lymphoid sheath (PALS), the germinal centers, and the marginal zone.
- The red pulp is composed of the splenic cords, vascular sinuses, and macrophages, lymphocytes, plasma cells, and erythrocytes.
There is no lymph supply to or from the spleen.
Broadly, think of the spleen as a screening and filtering organ. The blood is screened for pathogens and aged RBCs, and inappropriate organisms or material are filtered out.
3.2 Examination of the spleen
When examining the spleen, take care to note the size (enlarged vs normal vs small), the presence of nodules, any areas of discolouration, and any changes in texture. The spleen should be serially sectioned: cross-sectional slices approximately 1 cm thick made through the entire spleen from head to tail.
Small spleens are generally unimportant. Splenic aplasia is extremely rare, and unlikely to be encountered.
Large spleens (“splenomegaly”) are of greater concern and are far more common. While going through this section, you should be prepared to formulate lists of differential diagnoses for nodules within spleens and for diffusely enlarged spleens. Large spleens may be diffusely enlarged, or enlarged by single or multiple nodules. On cut-section, they may be bloody (i.e. ooze blood on cut section), or ‘meaty’ (i.e. enlarged by something other than blood). Although there is overlap between these phenotypes, broadly classifying spleens in this way can help narrow down a list of differential diagnoses ((Table 3.1. Note: the table has formatting issues in the PDF, and is easier to read online).
| Features | Nodular | Diffuse |
|---|---|---|
| Bloody | Hematoma, hemangiosarcoma, acute splenic infarct, incomplete splenic contraction | Congestion, splenic torsion, septicemia, hemolytic anemia |
| Meaty | Nodular hyperplasia, various sarcomas, metastatic neoplasia, granulomas | Hemophagocytic histiocytic sarcoma, leukemia/lymphoma, amyloidosis |
3.3 Circulatory disturbances
3.3.1 Congestion
With their enormous capacity to store blood, spleens can vary tremendously in size. Congestion leads to an enlarged spleen that oozes blood on cut section. The most common cause of an enlarged, congested spleen is barbituate euthanasia, but splenic torsion, acute hemolytic anemia, or shock can also lead to a congested spleen.
3.3.2 Hematoma
Hematomas are common, benign, non-neoplastic masses found in the spleen, particularly of dogs. They can range greatly in size, from 1 - 2 cm up to very large, 20 - 30 cm masses. They are dark red, poorly demarcated from the adjacent splenic parenchyma, and are bloody on cut section. They may be caused by trauma, or by alteration of the circulatory system secondary to other benign growths, such as nodular hyperplasia. Rupture of theses masses can lead to hemoabdomen and hypovolemic shock. These masses cannot be grossly differentiated from hemangiosarcoma, and thus must be submitted for histopathology.
3.3.3 Necrosis and infarction
Infarctions are common lesions found in the spleen. Due to the nature of the vascular supply of the spleen, there is little collateral circulation, rendering them prone to infarction. Acute venous infarcts (Fig 3.1) generally appear bright red, well demarcated, and may bulge slightly on cut section. As the infarct matures, the necrotic tissue is removed and replaced by fibrous connective tissue (Fig 3.2), and becomes pale and firm (in other words, the spleen develops a scar).
Figure 3.1: Irregularly arborizing areas of deep red are noticable along the surface of this spleen, consistent with acute necrosis.

Figure 3.2: Along the left lateral aspect of this cross-section of a spleen, there is a pale white, well demarcated area consistent with a chronic infarct
Although splenic infarctions are often incidental findings, they may be key lesions in certain diseases. Infectious diseases causing vascular damage, for example classical swine fever virus or sepsis, can lead to infarction. Hypercoagulable states, seen with immune-mediated hemolytic anemia or as a paraneoplastic syndrome, can result in infarction. Endocarditis (bacterial infection of the heart valves) can result in septic emboli or in thrombi, which can lodge in the small vessels of the spleen and lead to infarction.
3.4 Inflammation and infection
Unlike some other tissues (for example, liver or lung), infections only rarely settle and become chronic in the spleen, perhaps due to the large numbers of phagocytic cells. The spleen, however, can react to blood-borne pathogens, or to pathogens that target lymphoid tissues. A few examples of each are discussed below.
3.4.1 Sepsis
Given its role in filtering the blood, it is understandable that the spleen would react to massive blood-borne infections of sepsis. In some instances of sepsis, the spleen is markedly enlarged by blood, soft, and dark red.
3.4.1.1 Anthrax
Anthrax is important not because it is a frequent disease (its rare), but because it is fatal to many animals, including humans (it’s also reportable). It is caused by Bacillus anthracis, a gram-positive, spore-forming bacteria. Herbivores in particular are susceptible, while reptiles and carnivorous birds are resistant. Infection is generally acquired through the disturbance of soil (e.g. excavation), which exposes the spores that are highly resistant to the environment, and can live for long periods (years) in adverse conditions. Cattle and sheep ingest spores, and spores enter circulation through traumatized mucous membranes. Carnivores may become infected by consuming the carcass of an infected herbivore, or through direct consumption of the spores. Following ingestion, the spores germinate and traffic to lymph nodes, causing a lymphangitis and lymphadenitis that then progresses to septicemia. The bacteria produce 3 key toxins: edema factor, lethal factor, and protective antigen. These toxins contribute to vascular damage and impaired coagulation, along with injury and inactivation of phagocytes. Death is usually rapid (< 24 hours), especially in sheep and goats. Cattle may also die rapidly, but some probably recover.
The classical presentation of a case of bovine anthrax is sudden death of the animal with little to no clinical signs. The animal will often ooze blood from its orifices, and will appear to have putrified more rapidly than expected. Marked splenomegaly is common, and blood fails to clots.
There are 2 reasons why you will hopefully never see the splenomegaly associated with anthrax. 1) It is a rare (and zoonotic!) disease. 2) You should not necropsy an animal you suspect is affected with anthrax.
To elaborate on point 2: massive replication of B. anthracis in the host occurs prior to death. Upon exposure to air, the organisms sporulate, forming the highly resistant organisms that can contaminate the environment. Thus, instead of necropsying these animals and getting anthrax spores all over the place, a blood smear taken prior to necropsy can identify the organisms. Rapid body-side test kits for B. anthracis are also available. Animals that are confirmed positive for B. anthracis are incinerated.
3.4.2 ASFV and CSFV
African swine fever (ASF) virus and classical swine fever (CSF) virus are two reportable diseases of swine. They both affect a variety of organs, and the spleen is one. Both diseases cause lymphoid depletion. CSF often leads to hemorrhagic infarcts, while ASF causes marked splenomegaly.
3.4.3 Chronic splenitis
This is relatively uncommon. Granulomas, presenting either as multifocal pale nodules or a diffusely pale spleen, can be caused by Mycobacterium spp. and systemic fungal diseases such as histoplasmosis or blastomycosis.
Splenic abscesses are similarly uncommon. Rhodococcus equi (in horses) is perhaps the most common agent, along with Truperella pyogenes (cattle).
3.5 Hyperplasia and neoplasia
Neoplastic diseases of the spleen present either as nodules or as a diffusely enlarged, meaty (but not bloody) spleen. The former is more common. It is not possible to differentiate grossly between nodular lesions on the spleen, biopsy and submitting them for histopathology is the only way to diagnose these masses.
The most important neoplasm of the spleen is hemangiosarcoma.
3.5.1 Nodular hyperplasia
Nodular hyperplasia is a common finding in the spleens of dogs, and to some degree bulls. Nodular hyperplasia simply refers to the proliferation of elements of the spleen to the extent that they form a nodule. The elements may be lymphoid in origin (lymphoid nodular hyperplasia), may be foci of extramedullary hematopoeisis, or may have a mixture of varying components. They are benign lesions, but can be difficult to grossly differentiate from hemangiosarcoma. They are usually small (up to 2 cm, but can be larger), and often have irregular areas of palor on cut-section. They may be a predisposing factor for the development of a splenic hematoma.
3.5.2 Hemangiosarcoma
Hemangiosarcoma is the most common malignant neoplasm of the spleen in dogs, and is almost invariably fatal. There is no cure. The cause of the disease is uncertain, but likely multifactorial.
The gross appearance of hemangiosarcoma is variable (see Fig 3.3). The neoplasm may appear as one or multiple nodules, some of which may be ruptured. It is not uncommon for a portion of the omentum to adhere to ruptured nodules. Nodules are generally diffusely red or mottled red and white, bloody on cut section, and are poorly demarcated from the adjacent spleen. Larger nodules are often necrotic at their core.
Figure 3.3: A large, red, friable, partially necrotic mass is present at the approximate midpoint of the spleen. Multiple other variably sized, red, raised masses are present randomly throughout the remaining parenchyma.
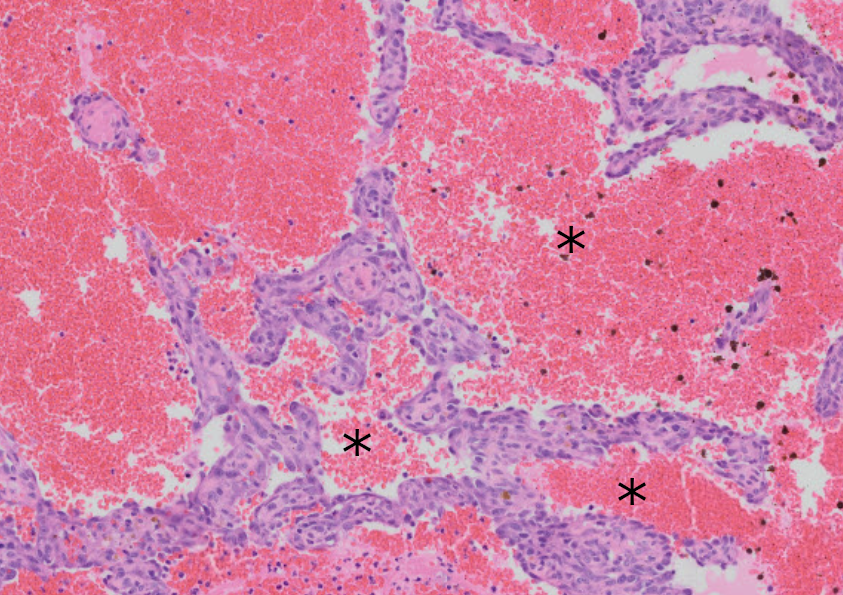
Figure 3.4: Hemangiosarcomas are composed of channels (marked with *) filled with blood and lined by neoplastic endothelial cells. Note the thin walls of these channels: these masses are fragile and rupture easily, leading to hemorrhage both within the mass and potentially into the abdomen.
Histologically, hemangiosarcomas are composed of thin-walled, endothelial-lined channels filled with blood (Fig 3.4. As you can imagine, these channels are fragile, and one of the most common and serious complications of hemangiosarcoma is rupture, leading to variably profound hemorrhage (hemoabdomen), shock, and/or death.
Hemangiosarcomas are very prone to metastasis, and frequently metastasize to the liver and lungs (Fig 3.5), as well as the heart and CNS. In the liver and lungs, metastatic nodules are distinctive, small (~ 1 cm), raised, spherical, red masses, colloquially referred to as “cannonball” lesions. They can also metastasize to the mesentery, where they must be differentiated from splenosis.
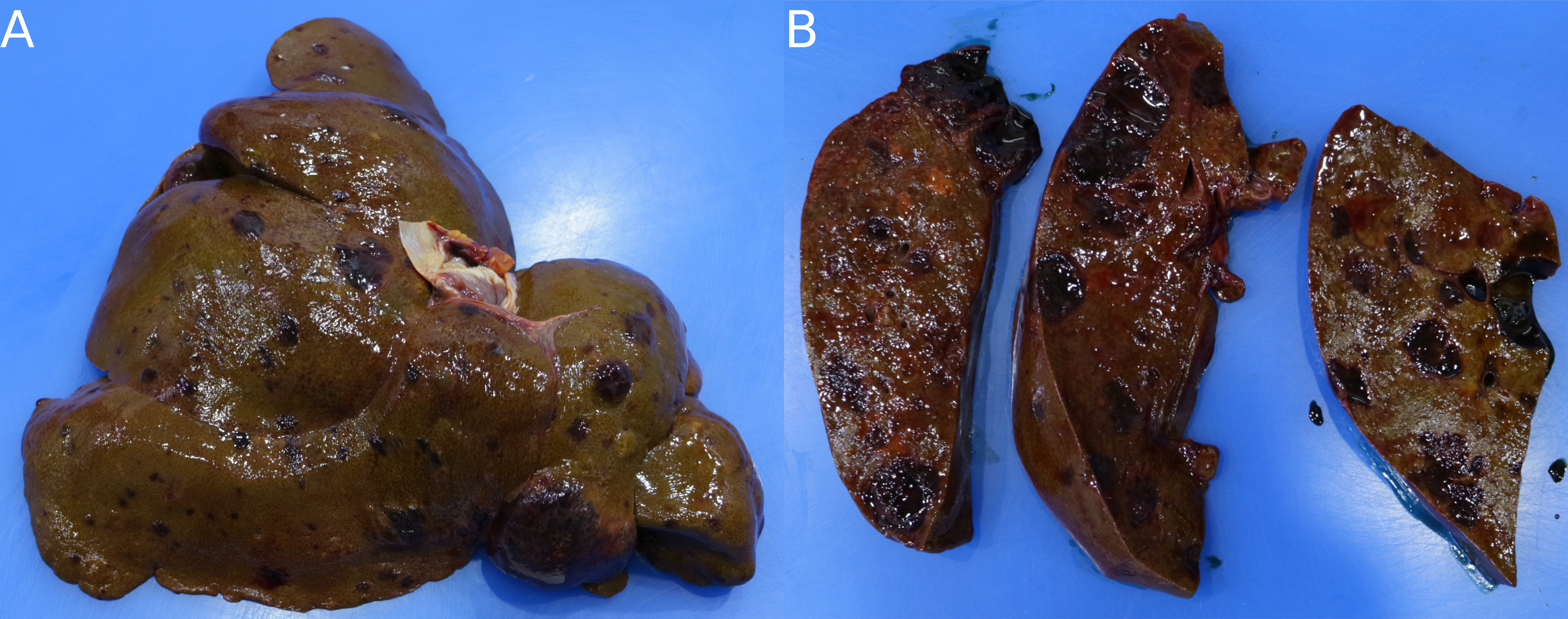
Figure 3.5: A) A canine liver with multiple, variably sized, raised, roughly spherical masses protruding from the capsular surface. B) On cut section, the masses are red, geletinous, well-demarcated, and roughly spherical.
3.5.3 Lymphoma
Lymphoma in the spleen is similar to that in other locations (see main section on Lymphoma). There are uncommon forms of primary splenic lymphoma that are generally slow growing and tend to be limited to the spleen (e.g. mantle zone and marginal zone lymphomas), but these will not be covered here. Various leukemias can also enlarge the spleen.
3.5.4 Hemophagocytic histiocytic sarcoma
This is a malignant neoplasm that often manifests with splenomegaly. It is characterized by a diffusely meaty spleen, enlarged by huge numbers of neoplastic macrophages. These macrophages phagocytose erythrocytes, leading to anemia, often mimicing immune-mediated hemolytic anemia. It is primarily a neoplasm of the dog, and may also affect the bone marrow.
3.5.5 Non-angiomatous, non-lymphomatous sarcomas
This is a catch-all term for the large variety of other tumours that can arise in the spleen. They are grouped together because they share a similar gross and histological appearance, and, more importantly, a similar clinical behaviour. They can be broadly divided into two categories: clinically benign tumours, and their nefarious counterparts, malignant and metastasizing masses (Fig 3.6). Mitotic rate is the most useful histologic feature in differentiating between the two. The survival time for animals with tumours of a high mitotic rate is greatly decreased as compared to those with low mitotic rates. The liver is the most common site of metastasis.
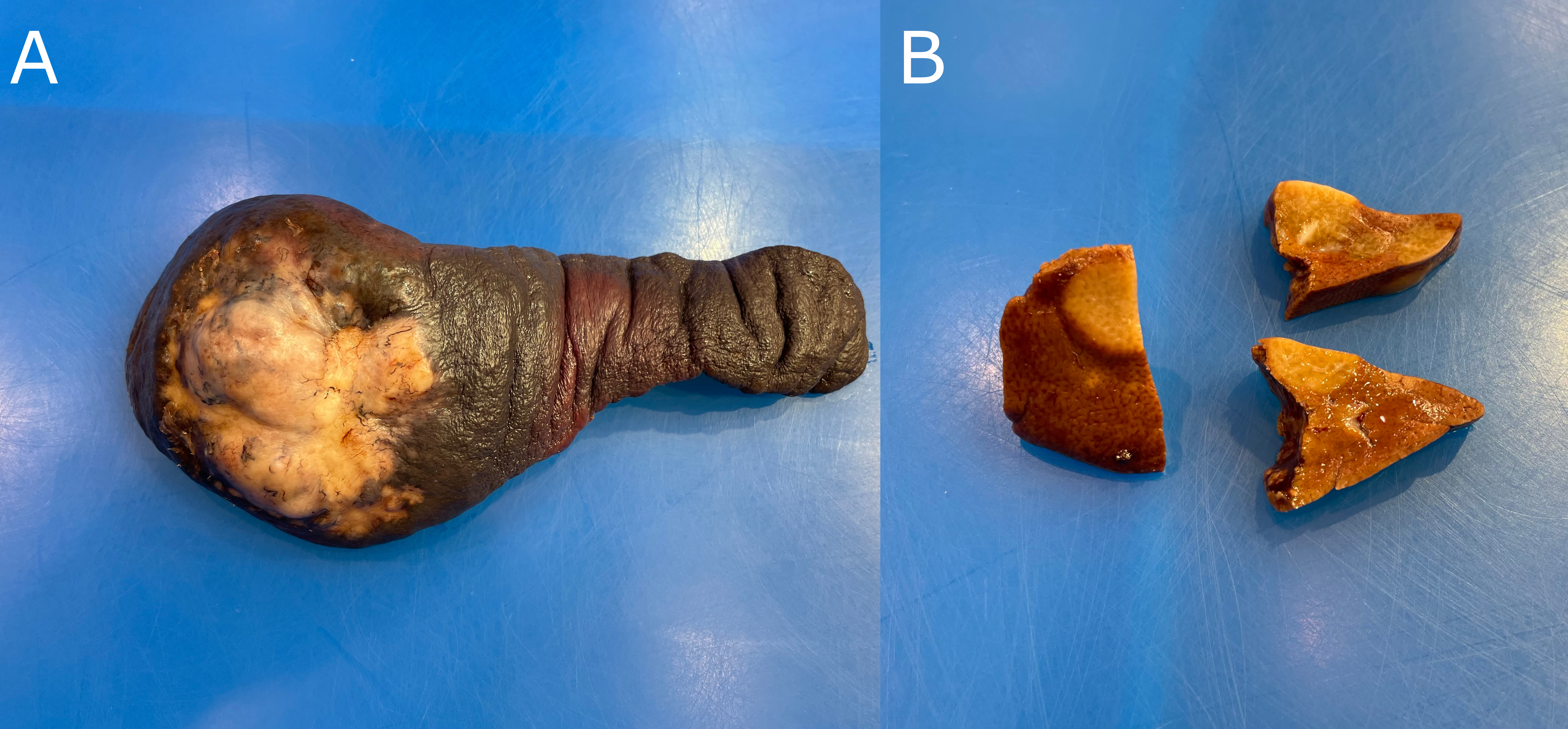
Figure 3.6: A) A canine spleen with a single, raised, firm, white, multilobular, poorly demarcated mass. B) A mass of similar description is present within multiple samples of liver (suggestive of metastasis).
3.6 Miscellaneous conditions
3.6.1 Siderotic plaques
Syn: Gamna-Gandy bodies, siderofibrotic plaques.
These are very common lesions in the spleen of geriatric dogs, and are thought to be the result of previous trauma or hemorrhage. They are found in the splenic capsule, typically along the periphery but often extending centrally, and are tan to brown to yellow, firm, and dry. Their primary importance is in recognizing that they are incidental findings, of no clinical relevance.
3.6.2 Splenic rupture
Rupture of the spleen can be an acute, life threatening event, primarily due to massive hemorrhage and hypovolemic shock. Prompt surgical intervention is generally required. By far, the most common cause of splenic rupture is trauma. Occasionally, splenic rupture is diagnosed in the absence of trauma; in those cases, an underlying cause for abnormal splenic fragility should be sought. In particular, neoplasms or other growths may render the spleen more susceptible to rupture.
An interesting and occasional consequence of splenic rupture is the so-called “seeding” of the abdomen with splenic tissue, which can implant and develop functional capabilities. The presence of multiple small splenic nodules throughout the abdomen is known as splenosis. Be cautious, however, when you are faced with interpreting this lesion: metastatic hemangiosarcoma can also present as small red nodules throughout the mesentery, and they are very difficult to distinguish grossly.
3.7 Biopsying the spleen
Finally, a quick note on biopsies of the spleen. When a lesion is discovered on the spleen, the usual clinical course is a complete splenectomy. The entire spleen is always the best sample to send, however, it is not always technically feasible. In cases where the whole spleen cannot be sent, taking multiple samples of the junction of the mass (or lesion) and normal spleen is the most likely way to obtain a diagnosis. Many of these tumours, especially hemangiosarcomas, have necrotic centers, and sampling the mass alone may result in a dreaded “non-diagnostic” report. Note the word “multiple” above: the classical recommendation is that seven biopsies of a splenic mass are required to reliably rule out the possibility of hemangiosarcoma (though a current paper suggests that perhaps 5 are adequate).
Splenic masses, in particular, often come in multiples. They are not guaranteed to be the same thing. Therefore, each mass should be biopsied and submitted.
If a diffusely meaty spleen has been removed, send several 1 cm thick samples.