2 Bone marrow
2.1 Introduction
Bone marrow is found within the spongy portions of bones. It is the site of production of the cellular elements of blood, known as hematopoiesis, which can be broadly divided into myeloid and lymphoid components. The lymphoid lineage produces lymphocytes, while the myeloid lineage produces everything else (Fig 2.1). The massive number of cells produced by the bone marrow all originate from a common stem cell, known as the hematopoietic stem cell, which has the potential to differentiate into any type of cell type found in the blood. The first division commits a stem cell either to the lymphoid or myeloid lineage. Under the influence of growth factors and cytokines, they gradually differentiate as they divide, eventually becoming a fully differentiated, functional leukocyte, erythrocyte, or thrombocyte. Early stages of differentiation are morphologically indistinguishable, and the cells at this stage are often refered to as “blast” cells, short for the various names of immature cells (e.g. monoblast, myeloblast, rubriblast, etc.). The bone marrow is composed of cells of a variety of maturational stages, but mature, morphologically identifiable cells outnumber immature “blast” cells significantly.
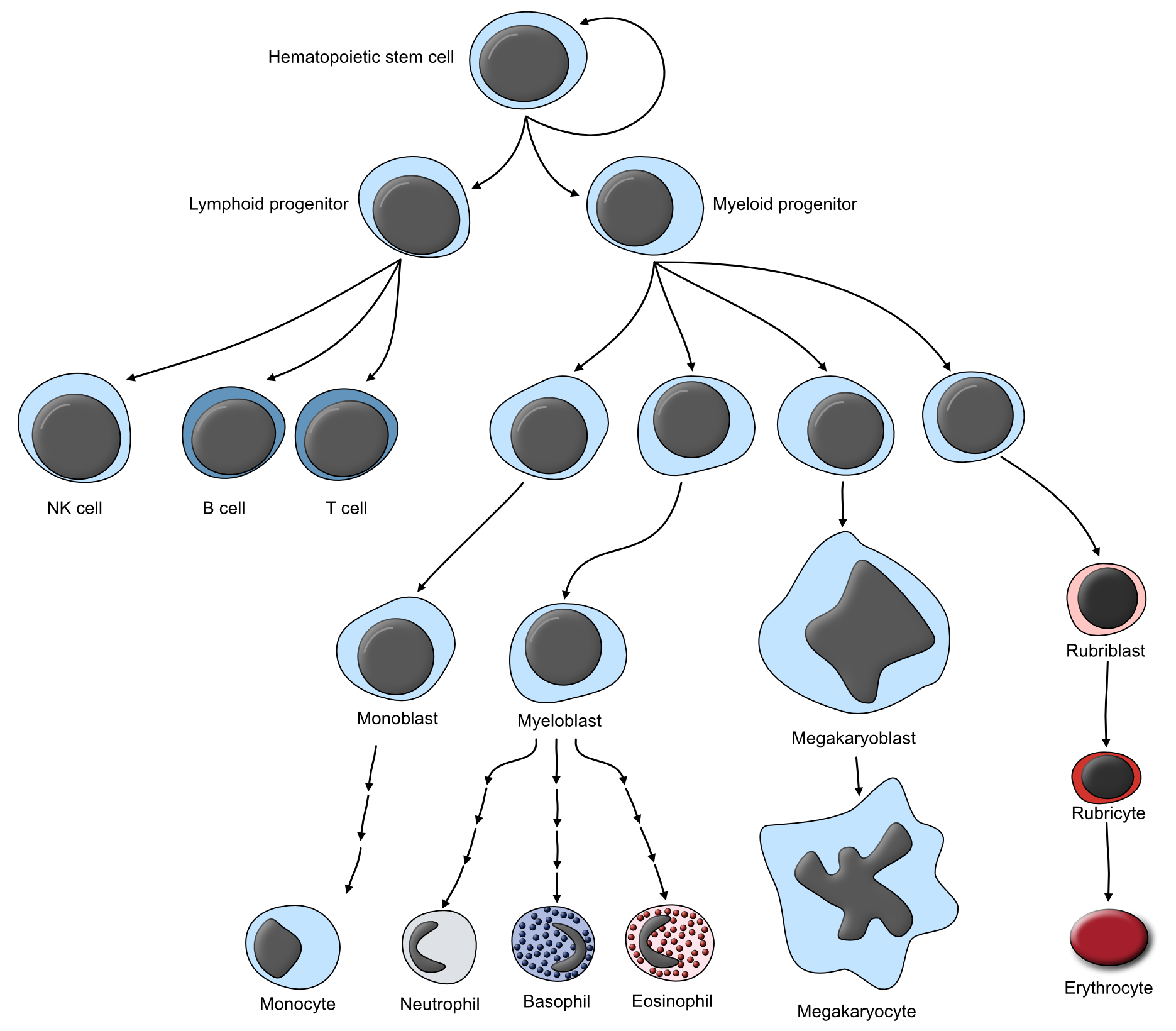
Figure 2.1: Schematic illustrating the progressive maturation of the various lineages of circulating blood cells.
The location of hematopoiesis changes as an animal grows. In utero, the primary sites of hematopoiesis are the liver and spleen. In neonatal and young animals, the marrow spaces of virtually all bones is occupied and generating cells. In adults, hematopoietic tissue is mostly restricted to axial bones (i.e. skull, vertebrae, ribs, sternum, and pelvis), as well as the proximal portion of the humerus and femur. As the hematopoietic tissue recedes during aging, it is replaced by fat. This has important implications when it comes to biopsying the bone marrow, as sites with active marrow must be sampled.
Because the products of hematopoeisis are easily evaluated by sampling the blood, biopsy or aspirate of the bone marrow is relatively uncommon. Similarly, because evaluation of the blood can reveal quite a bit about the underlying bone marrow, many of the diseases of bone marrow are better evaluated by clinical pathologists.
2.2 Adaptations of growth
2.2.1 Hyperplasia
Hyperplastic bone marrow is a common finding. The bone marrow responds to abnormally low levels of circulating cells by ramping up production of that particular cell type. Thus, many causes of anemia, inflammation, or thrombocytopenia can lead to marrow hyperplasia. Occasionally, the marrow cannot prodyuce the required cells. For example, the production of red blood cells (RBCs) requires iron-containing hemoglobin. In patients that are iron deficient, producing new RBCs is not possible, and thus hyperplasia cannot occur.
Grossly, the typically fatty portion of the marrow (i.e. the diaphysis of the long bones) changes from yellow to red. Hyperplasia of ANY cell line, not just the erythroid cell line, turns the marrow red.
2.2.2 Hypoplasia
Bone marrow hypoplasia refers to the decrease in or absence of production in one or more cell lines. Causes are numerous and varied. Lack of signals for growth and differentiation can lead to hypoplasia. For example, erythropoietin (EPO) stimulates differentiation and production of RBCs from the marrow. EPO is produced by the kidney. In cases of chronic renal disease, EPO production can decrease, leading to a concurrent decrease in the cells of the erythroid lineage (i.e. erythroid hypoplasia). Lack of nutrients can lead to hypoplasia of one or more cell lines. To continue the example from the section on hyperplasia, an absence of iron leads to an inability of the erythroid lineage to mature, and can result in hypoplasia. Degeneration and necrosis, which itself has a number of causes, can lead to bone marrow hypoplasia.
Grossly, hypoplastic bone marrow is reflected by an increase in the pale yellow component of the marrow and decrease in the red portion.
2.3 Degeneration and necrosis
Degeneration and necrosis of the bone marrow, depending on the extent, can have significant consequences for the animal. The most common presenting sign is a cytopenia in one or more cell lines. Recall that hematopoietic cells are metabolically and mitotically active: this renders them susceptible to a wide variety of insults. However, so long as the target of damage is not the hematopoietic stem cell (Fig 2.1), it is likely that the bone marrow will be capable of recovering, so long as the insult is removed.
Common causes of bone marrow damage include:
Damage from radiation
Infectious agents
- Feline immunodeficiency virus, feline leukemia virus, feline panleukopenia
- Equine infectious anemia virus
- Canine parvovirus 2
Immune-mediated diseases (e.g. immune-mediated hemolytic anemia)
Toxins and drugs
- Certain chemotherapeutic agents
- Idiosyncratic drug reactions
- Toxic substances
Idiopathic
2.4 Neoplasia
Before diving into this section, some explanation is needed. What is going to be discussed here are neoplasms that arise primarily from the precursor cells within the bone marrow itself, namely leukemias. A broader classification system uses the umbrella term “hematopoietic neoplasia” to define neoplasms that arise from any of the formed elements of blood. Thus, for example, a cutaneous mast cell tumour would be considered a hematopoietic neoplasm, because it is caused by a cell originally from the bone marrow, even though it forms a tumour in the skin. These types of tumours will not be discussed here, but will be discussed in other sections. A list of tumours not discussed here includes:
- Mast cell tumours (skin or GI)
- Plasmacytomas (skin)
- Cutaneous histiocytomas (skin)
Lymphoma is a very important disease, and is discussed in the section on lymph nodes, though it could arguably be included here.
Broadly, neoplasms of the marrow are separated based on their lineage of origin. Neoplasms of lymphoid origin include lymphoid leukemias, lymphoma, and plasma cell tumours. Neoplasms of the myeloid lineage include myeloid leukemias, myelodysplastic syndrome, histiocytic tumours, and mast cell tumours.
2.4.1 Myeloid neoplasms
2.4.1.1 Acute myeloid leukemia (AML)
AML is defined as an acute cytopenia accompanied by > 20 % blast cells in either the blood or bone marrow (or both). Recall that blast cells are immature cells that cannot be reliably identified by cytology or histopathology (in other words, it is a precursor cell that has not differentiated enough to have recognizable features). Cytopathologists are best suited to evaluate the number of blast cells, while histopathology of the bone marrow is useful to characterize the extent of myelopthisis (replacement of normal marrow elements by other tissues), and/or necrosis and fibrosis. In an ideal world, the type of AML could be further categorized by looking for specific differentiating immunohistochemical markers on the neoplastic blast cells; in reality, the cost is still high, and the current utility of doing so in veterinary medicine is debatable.
Animals with AML typically present with significant acute, non-specific illness. Routine bloodwork often reveals cytopenia of one or more cell lines that cannot be explained. Peripheral blood may or may not contain blast cells, in which case evaluation of the bone marrow by cytology or histopathology needs to be pursued. The liver and/or spleen may be diffusely enlarged in some cases. The prognosis for animals with AML is very poor.
2.4.1.2 Myelodysplastic syndrome
Myelodysplastic syndrome is similar to AML and presents primarily in cats and dogs as an acute, non-specific illness. Bloodwork demonstrates one or more non-regenerative cytopenias and dysplasia of cells either in the blood or bone marrow. The bone marrow contains an increased proportion of blast cells, between 5 - 20 %. A variety of subclassifications exist. Non-regenerative anemia is frequently present. In dogs, MDS often preceeds AML.
2.4.1.3 Myeloproliferative neoplasms (chronic leukemia)
This is a very rare condition in animals, and is characterized by the slow accumulation of very large numbers of well-differentiated cells of the myeloid lineage. It is typically an incidental finding on routine bloodwork in older animals. Very large numbers of erythrocytes, thrombocytes, or various leukocytes may be noted in the blood. Other peripheral cytopenias are rare, and the bone marrow is not necessarily affected.
2.4.2 Lymphoid neoplasms
2.4.2.1 Acute lymphoid leukemia (ALL)
ALL is the lymphoid equivalent to AML. It is characterized by > 20 % of lymphoid blasts in the circulation and/or bone marrow. One or more cytopenias are usually noted on CBC, and there may be involvement of the liver or spleen. Feline leukemia virus is a predisposing factor in cats. In dogs, B-cell ALL is more common than T-cell. In dogs, ALL can enlarge lymph nodes, and it can be difficult to distinguish from a specific subtype of lymphoma.
Clinical signs are vague and non-specific, but are usually acute in onset. Prognosis is very poor.
2.4.2.2 Chronic lymphoid leukemia (CLL)
This is the most common type of leukemia in dogs. Most are of T-cell origin. This is an indolent form of leukemia, with a slow accumulation of lymphocytes either within the bone marrow (B-cell origin CLL) or spleen (T-cell origin CLL). Eventually the disease manifests with a profound lymphocytosis. Prognosis is fair to good, with most animals living 1 - 3 years after the diagnosis.
2.4.2.3 Multiple myeloma
Multiple myeloma is a malignant tumour of plasma cells, usually found in older animals. Dogs, and less frequently horses and cats, are affected. Tumours usually appear within the medulla of bones with active bone marrow, frequently the vertebrae and pelvis, leading to pain, weakness, or paresis. The tumours are osteolytic. As you might expect from a plasma cell tumour, the neoplastic cells secrete large quantities of a clonal immunoglobulin, which can be easily detected on routine bloodwork as a hyperglobulinemia. With electrophoresis, it can be determined that it is a monoclonal gammopathy. Proteinuria is also common, as the tumour produces free immunoglobulin light chains that freely pass through the glomerulus, known as Bence-Jones proteins. The diagnosis of multiple myeloma requires identification of not just the neoplastic plasmacytoid cells within the bone marrow, but also monoclonal gammopathy, Bence-Jones proteinuria, and osteolysis.
In cats, osteolysis is not common, and the tumour more often appears in the abdominal organs or skin.
2.5 Biopsy of the bone marrow
Marrow biopsy is somewhat rarely performed in daily clinical practice. There is nothing preventing you from biopsying the marrow, but it is difficult. You need proper equipment and technique. The relatively uncommon need for biopsy leads to clinician discomfort with a procedure they rarely perform. Along those lines, many cases with indications for bone marrow work-up end up being referred to specialty centers, where specialists end up performing the biopsies.
Work-up of the bone marrow should always include three submissions: 1) bone marrow biopsy, 2) bone marrow aspirate, and 3) CBC from the time of biopsy. Any historical CBCs should be also made available to the pathologists.
Biopsies are routinely taken from the humerus, femur, or ilium in small animals. In large animals, the sternum and tuber coxae are preferred sites. Aspirates and biopsies can be performed simultaneously. Bone marrow aspirates are delivered to clinical pathologists, who can best characterize and classify the aspirated cells. Core biopsies are sent to anatomic pathologists, who can identify architectural disturbances and the presence of abnormal marrow components, such as myelofibrosis, neoplasia, or inflammation.
A note on terminology: This is an “FYI” part of the notes, but may be useful to know. The terminolgy surrounding the evaluation of bone marrow has changed in recent years. One of the important metrics used to evaluate bone marrow is the ratio between cells of the granulocytic and macrophage lineages vs. those in the erythrocytic lineage. Historically, this was referred to as the “myeloid:erythroid” ratio (M:E ratio). This is inaccurate, however, as techinically both erythroid and granulocytic cells are part of the myeloid lineage. The terminology has now shifted, such that this evaluation is reported as the “granulocytic:erythroid ratio”. If you see the older, “M:E” term in the literature, it is referring to the same thing as the “G:E” ratio. Fascinating stuff. Refer back to Fig 2.1 for help with these terms.