3 Pathology of the cornea
3.1 Corneal ulcers
Corneal ulcers - the erosion of the surface epithelium with exposure of the underlying corneal stroma - are extremely common and occur for a variety of reasons. A few specific etiologies are dealt with below, however, there are some generalities that can be applied to most corneal ulcers, regardless of cause.
Recall that the corneal stroma is kept in a dehydrated state: this is accomplished by having an impermeable epithelial surface, as well as active pumps along the corneal endothelium (explained further Ocular anatomy). Thus, any break to the epithelial barrier will result in stromal edema – through the absorption of tear film – that manifests clinically as a blue-ish tinged cloudiness of the cornea. Neutrophils found in the tear film can also enter the stroma through the disrupted barrier.
What happens next is dependent on the degree of corneal injury. If the injury is relatively minor – for example, the erosion of the superficial epithelium and perhaps a small amount of the stroma – then the remaining epithelium can slide over the defect, providing an initial, thin barrier. Mitosis begins within 24 hours so that the epithelium will soon return to its normal thickness. Be aware, however, that for healing to be succesful, the epithelium must be able to adhere to the underlying stroma. Some cases of refractory corneal ulcers occur due to the presence of degenerative superficial stroma that fails to provide an adequate foundation onto which the epithelium can anchor. Procedures that remove the uncooperative tissue (e.g. grid keratectomy) allow new granulation to form, thereby providing a scaffold for the epithelium to anchor to.
Deeper wounds – those with more than 1/3 of the stroma affected, give or take – require a bit more investment in healing. Epithelial sliding cannot occur in these cases before the stroma is rebuilt. To rebuild corneal stroma, the keratocytes (fibrocytes of the corneal stroma) undergo fibroblastic differentiation and begin producing ground substance. Fibroblasts and small blood vessels also begin migrating from the limbus, and it is these fibroblasts that are the primary source of collagen. This migration takes time. Approximatley 4 days to get started, then about 1 mm every day thereafter. The new cornea is never quite the same as the original. Over time, the newly deposited collagen will organize to resemble normal stroma, and the tissue will become less cellular, but a scar will be detectable. The degree to which a scar affects vision is dependent on the initial insult (fun fact: I have a scar across my cornea, courtesy of an errant hockey stick. It doesn’t obstruct my vision at all). The epithelial component of a larger wound is also different: in large defects, epithelial reserve cells from the limbus are recruited, and these cells have a conjunctival, rather than corneal, phenotype, and may be pigmented, though if the injurius stimulus is removed, these epithelial cells will gradually become more “corneal”.
Ulcers that progress may erode through the entire corneal stroma all the way to Descemet’s membrane. In these cases, Descemet’s membrane may protrude through the corneal defect, creating a small structure known as a Descemetocele 3.1. These cases are clinical emergencies, as the membrane may rupture at any time, resulting in a full corneal rupture with significant adverse consequences. A ruptured cornea will repair with fibrin, but occasionally the iris may prolapse into the defect, forming an anterior synechia - an irreversible change.
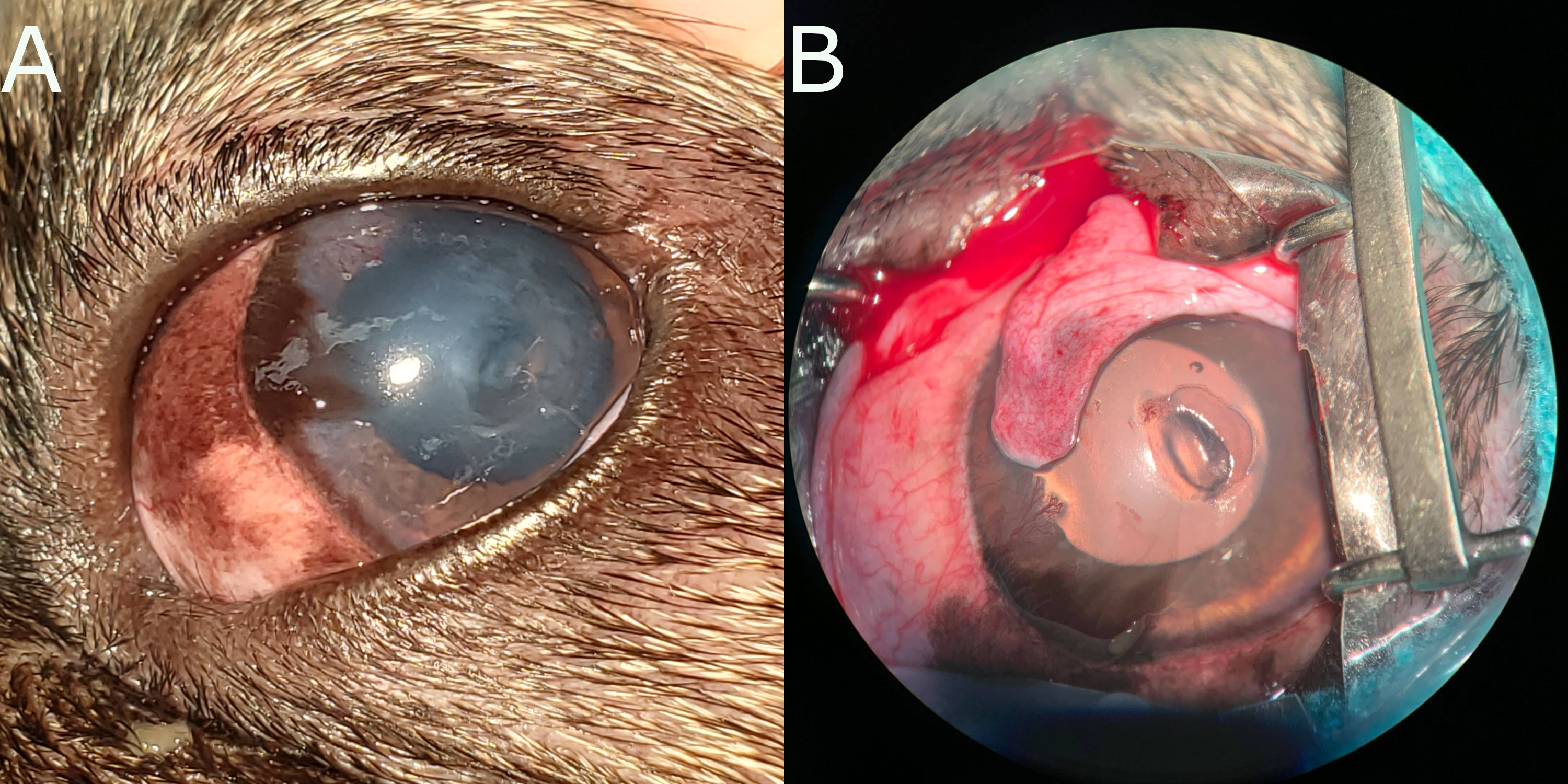
Figure 3.1: A) A descemetocoele in the cornea of a dog. Note the deperessed area just below center. The cornea is cloudy, due to corneal edema, and there is some pigmentation of the corneal epithelium. B) Intraoperative photograph of the same descemetocoele. The lesion has been debrided (i.e. dead corneal epithelium and stroma has been removed), resulting in the larger appearance. Note that the periphery fo the descmetocoele appears shallower - this illustrates how the tissue surrounding the deep ulcer was also affected, and would have delayed healing. Photographs courtesy of Alanna MacKinnon (AVC 2021).
Further complicating matters is whether the injury is sterile or infected. Sterile injuries tend to heal more quickly, while those that are complicated by infectious agents recruit more neutrophils, which induce bystander damage by lysing the corneal stroma (keratomalacia), and may delay healing, or even worse, lead to rapidly progressing corneal damage in the form of a melting ulcer.
3.2 Keratoconjunctivitis sicca
Keratoconjunctivitis sicca (KCS) is a condition in which the cornea desiccates (dries out), usually due to a decreased quantity of tear film, and rarely due to an altered quality of tear. The condition is most common in dogs, in which it is progressive and chronic. The etiology is unknown, but the success of immunosuppressive therapy suggests an autoimmune basis.
The lesions depend on the degree to which tear film production is affected. Most cases are moderate in onset, and result in chronic injury to the cornea, manifesting as cutaneous metaplasia. As tear film decreases, injury becomes more severe, and corneal ulcers may develop. There is nothing distinctive about these ulcers, with the sole exception that corneal edema may be minimal (as there is no tear film providing water to the cornea).
The condition is readily treated with artificial tears and immunosuppressive therapy.
3.3 Fungal keratitis
Fungal keratitis is a particularly common and important issue in horses. It is usually iatrogenic, resulting from the treatment of a routine corneal ulcer with antibiotics, and especially corticosteroids. Colonization of the wound by fungi (usually Aspergillus) results in a deep ulcerative keratitis and is accompanied by suppurative keratomalacia that is refractory to treatment with antibiotics. For unknown reasons, the fungi are often found deep in the cornea near Descemet’s membrane. This unusual distribution explains why superficial sampling – either corneal scrapes or corneal biopsy – may miss the fungi, and produce false-negative results. Do not be surprised, therefore, when a pathology report from a horse with suspected fungal keratitis informs you that although no organisms were seen, the condition cannot be ruled out!
3.5 Infectious bovine keratoconjunctivitis
Infectious bovine keratoconjunctivitis, also called “pinkeye”, is caused by Moraxella bovis. Along with Conjunctival squamous cell carcinoma, it is the most important ocular condition of cattle.
Cattle are infected by Moraxella bovis via fly vectors, and thus the disease is more common during the summer months. Upon infection, the bacteria adhere and then invade into the corneal epithelium, resulting in a small corneal ulcer accompanied by corneal edema and hyperemia. If left untreated, the ulcer will enlarge, deepen, and become infiltrated by large numbers of neutrophils. Damage from the indiscriminate neutrophils results in keratomalacia. In most cases, healing occurs as the necrotic cornea is shed and granulation tissue fills the wound, eventually scarring over. Despite the often relatively large ulcer found in the acute stages of the condition, the final scar is often small and clinically insignificant.
3.6 Dystrophies and depositions
The accumulation of material within the cornea is relatively frequently seen in clinical practice. It may occur as dystrophies or deposits.
A dystrophy is an inherited, bilateral, defect in structure or function of one of the components of the cornea. It is not triggered by injury or disease, either directly to the eye or elsewhere in the body. The most common, which are seen in dogs, are lipid and crystalline dystrophies. In the case of the former, cholesterol is deposited in the corneal stroma, while in the later mineralization may be present in the superficial corneal stroma.
Corneal deposits typically occur secondarily to metabolic disease or injury. Metabolic deposits include mineral, lipid, or pigment. The most common metabolic deposit, again seen in dogs, takes the form of corneal lipid associated with hyperlipidemia of any cause - though often associated with Cushing’s disease, diabetes mellitus, or hypothyroidism.
Corneal deposits secondary to injury are again most common in dogs. Melanin deposits occur in corneas subject to chronic irritation.
](images/dendritic_ulcer_feline.jpg)