1 Introduction
1.1 The anatomy of muscle
The basic structural unit of muscle is the myofiber, which represents a single, long, tubular cell (Figure 1.1). Within each myofiber are many tightly packed myofibrils, composed of actin and myosin filaments (myofilaments), and which form the contractile machinery of the muscle. It is the arrangment of myofibrils that form the striated appearance of skeletal muscle that can be appreciated under light microscopy (Figure 1.2). The cytoplasm of a myofiber is known as the sarcoplasm, and the cell membrane is called the sarcolemma. Each myofiber is surrounded by a small amount of connective tissue called the endomysium. Multiple myofibers form a fasicle that is surrounded by another layer of connective tissue, the perimysium. Finally, multiple fasicles group together to form a muscle, which is surrounded by the epimysium.
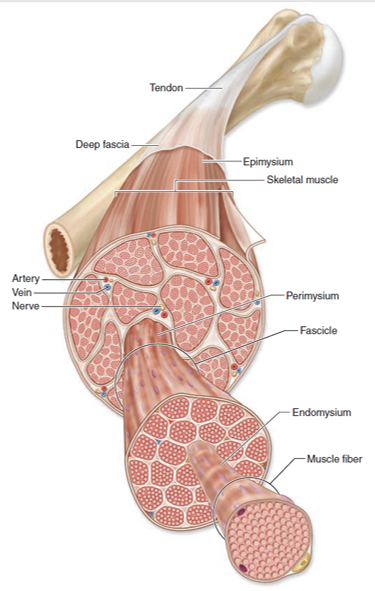
Figure 1.1: Structure and anatomy of muscle
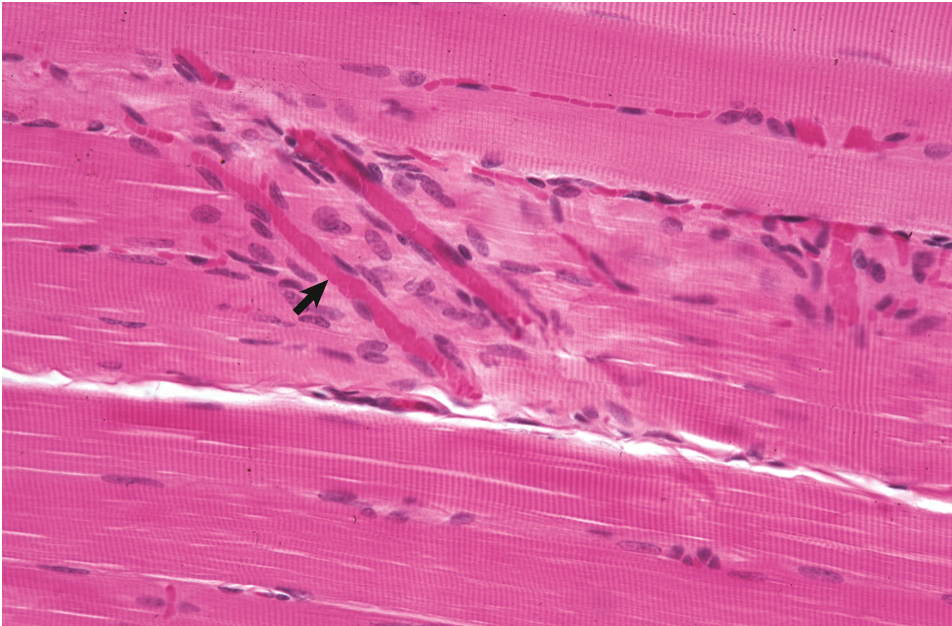
Figure 1.2: Normal skeletal muscle demonstrating striations. The arrow indicates a small capillary. Figure from Zachary and McGavin.
Skeletal muscle is characteristically multinucleated: each myofiber has hundreds of nuclei scattered along its length, almost always found along the periphery of the cell. Each nucleus within a myofiber controls a specific portion of the myofiber, and each nucleus acts independently. Nuclei within muscle fibers are terminally differentiated, meaning they can no longer divide, thus limiting the capacity of muscle for regeneration. Having multiple nuclei within a cell provides a distinct and somewhat unique benefit: localized damage, affecting a small number of nuclei, will not necessarily kill the entire cell (myofiber), and provides the myofiber with an opportunity to regenerate. We will discuss muscle regeneration in more detail in the section on Necrosis and regeneration.
Closely associated with individual myofibers are satellite cells. The nuclei of satellite cells are indistinguishable from myofiber nuclei under light microscopy. Satellite cells are a type of stem cell, and are important in muscle regeneration and repair.
Myofibers can be subclassified to reflect their function. The classification is based on three physiologic properties:
- Rate of contraction (fast vs. slow)
- Rate of fatigue (fast vs. slow)
- Type of metabolism (oxidative, glycolytic, or mixed)
Taking these characteristics into consideration leads to three subtypes: Type 1, Type 2a, and Type 2b (Table 1.1). Note that muscles are rarely, if ever, composed of a single subtype: they are a mixture of the different subtypes, though one type often predominates.
A basic review of the innervation of muscles is also worthwhile. Every myofiber is innervated by a motor neuron, and each motor neuron typically innervates multiple myofibers. The number of myofibers innervated by a neuron is dependent on the need for fine control: only 1-4 myofibers of the occular muscles, for example, are innervated by a single neuron, compared to the quadriceps where 150 or more myofibers may be innervated by a single neuron.
| Fiber type | Physiologic properties | Morphologic properties | Examples |
|---|---|---|---|
| Type 1 | slow twitch, fatigue resistant, oxidative, aerobic, ‘red’ | High mitochondrial and fat content, low glycogen | Muscles involved in prolonged activity, e.g. postural muscles, diaphragm |
| Type 2a | Fast twitch, oxidative, glycolytic, fatigue resistant | Intermediate mitochondria, fat, and glycogen content | |
| Type 2b | Fast twitch, fatigue sensitive, glycolytic, ‘white’ | Low mitochondrial and fat content, high glycogen | Muscles involved in athletic acitivty, e.g. sprinting |
1.2 The function of muscle
The contraction of muscle is a complex, orchestrated process in which myofilaments (the components of myofibrils) undergo a conformational change. The contraction of muscle is initiated at the motor end plate by the release of acetylcholine from a motor neuron into the neuromuscular junction. This depolarizes the myofiber, resulting in the release of calcium from the sarcoplasmic reticulum. It is the binding of calcium to the myofilament tropononin that results in the contraction of the sarcomere (recall from physiology that the sarcomere is the functional unit of contraction). In order for muscle to then relax, calcium must be pumped back into the sarcoplasmic reticulum in an ATP-dependent process. The importance of ATP in muscle relaxation is highlighted by a common and well-known change encountered at post-mortem: rigor mortis. Muscles retain the ability to contract immediately after death, but as ATP is consumed, and not replaced, relaxation cannot occur. This leads to the hard, contracted, and immobile carcass characteristic of rigor mortis. Eventually, relaxation of the carcass occurs due to breakdown of muscle either from autolysis or putrefaction (bacterial decomposition).
1.3 Response of muscle to injury
Skeletal muscle can undergo a fairly limited range of changes in response to environmental and physiologic stimuli. Muscle can shrink (atrophy), get bigger (hypertrophy), or die (necrosis). Under specific circumstances, muscles that have been only mildly injured can regenerate. The reaction of muscle to injury tends to proceed in a fairly sterotypic fashion regardless of the inciting cause, making it difficult to determine the underlying etiology from gross or histopathological exmaination alone. Thus, it is important to provide a good clinical history when submitting a case with suspected muscle injury. Supplementary tests (special stains, culture, etc.) are helpful (and occasionally necessary) in obtaining a definitive diagnosis.
1.3.1 Atrophy
Atrophy simply refers to the reduction in volumne of myofibers and muscle, and provided the cause can be corrected, is usually reversible.
1.3.1.1 Denervation atrophy
Denervation atrophy is caused by the loss of a nerve that innervates a myofiber. It is rapid, severe, relatively common, and can result in the loss of more than half of the affected muscle mass in a matter of weeks. The maintenance of a normal myofiber diameter is reliant in part on an intact associated nerve. Motor neurons release trophic factors at the motor end plate, which are critical in maintaining normal myofiber mass. Loss of a nerve leads to loss of the trophic factors, resulting in atrophy. Interestingly, atrophy is not due to the lack of contractile activity: paralytic disorders such as Botulism that affect the neuromuscular junction do not lead to atrophy. Denervation atrophy tends to affect affect both type 1 and 2 myofibers. Because only myofibers innervated by the specifically affected neuron undergo atrophy, the remaining myofibers within a fascicle may undergo hypertrophy to compensate.
Examples of disorders caused by denervation atrophy include equine laryngeal hemiplegia, “Sweeney”, and radial nerve paralysis.
1.3.1.2 Disuse atrophy
Decreased contractile activity of a muscle for any reason leads to disuse atrophy. Common causes include lameness/pain or limb immobilization (e.g. cast). Disuse atrophy occurs more gradually then denervation atrophy. Disuse atrophy preferentially leads to atrophy of type 2 fibers, however, this is somewhat inconsistent, thus relying solely on type 2 atrophy to distinguish this from denervation atrophy is unreliable. Unlike dennervation atrophy, usually all myofibers within a muscle group are affected by disuse atrophy, and thus there is no compensatory hyertrophy.
1.3.1.3 Nutritional (malnutrition or cachexia) atrophy
Failure to supply the required level of dietary nutrients to maintain normal muscle mass causes nutritional atrophy. It is a gradual form of muscle loss and tends to be generalized, though in the dog, loss of the temporal, back, and thigh muscles is most prominent. Muscle proteins undergo continuous turnover, and in states of starvation, are used as a source of nutrients. Nutritional muscle atrophy is usually accompanied by fat loss.
Animals with chronic illness or neoplasia lose muscle mass due to increased circulating levels of tumour necrosis factor (TNF, also known as “cachectin”), which increases myofiber catabolism. Type 2 fibers are preferentially affected. The term “chachectic” refers to animals with muscle atrophy secondary to illness or disease, in spite of the provision of adequate nutrition.
1.3.1.4 Atrophy of endocrine disease
Atrophy of endocrine disease is most commonly noted in companion animals. In dogs, hypothyroidism leads to altered metabolism of carbohydrates and loss of energy, leading to atrophy. In canine hyperadrenocorticism, atrophy is caused by increased catabolism and inhibited synthesis of muscle proteins. Type 2 fibers are preferentially affected. In cats, hyperthyroidism can lead to muscle atrophy, with non-specific myofiber damage occasionally observed.
1.3.2 Hypertrophy
Hypertrophy refers to grossly enlarged muscles, or to histologically enlarged myofibers. It does not refer to an increase in number of myofibers. It can be the result of physiologic stimulation or pathologic processes. Physiologic hypertrophy is generally the result of increased workload on the muscle. Pathologic hypertrophy occurs in response to a number of conditions. Compensatory hypertrophy of unaffected myofibers can occur in a background of neuropathic atrophy.
1.3.3 Necrosis and regeneration
Myofiber necrosis is a non-specific finding that accompanies a variety of different diseases and conditions. Grossly, necrosis of muscle is usually only appreciated in moderate to severe cases. Necrotic muscle is typically pale pink to white, and may appear streaked and slightly gritty if mineralization has occurred (for example, in Selenium and vitamin E deficiency)(Figure 1.3). Necrotic muscle can alternatively appear deeply red, if hemorrhage has occurred concurrently.
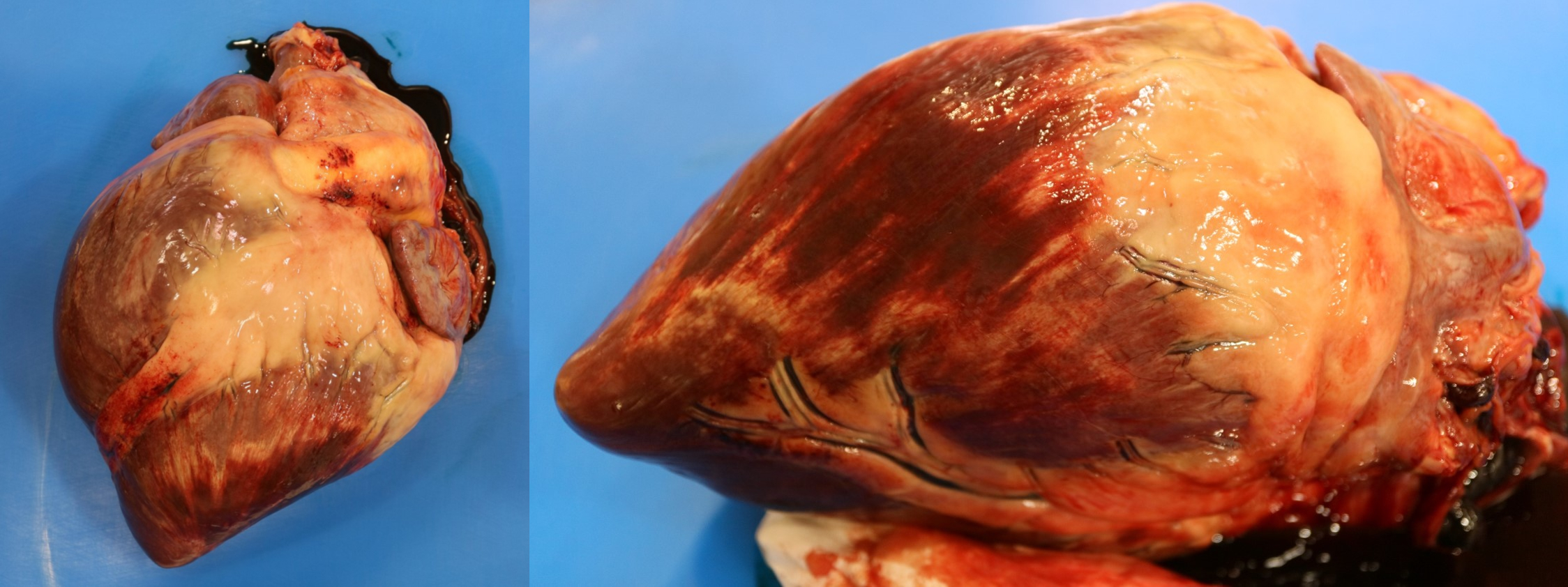
Figure 1.3: Necrosis of the myocardium. Pale white streaks bordered by hemorrhage are visible throughout the ventricle. Photo: C. Martin
The outcome of muscle damage and necrosis is dependent on its severity. A structure known as the basal lamina plays an important role. The basal lamina is a thin layer of extracellular matrix that keeps satellite cells closely associated to the myofiber, and just importantly, keeps fibroblasts out. If the basal lamina remains intact, and the damage only affects a small portion of the myofiber, then muscle can regenerate. These two criteria (intact basal lamina, focal area of damage) determine whether or not a muscle will regenerate or undergo fibrosis.
Although myofiber nuclei themselves cannot divide, recall that each myofiber is accompanied by satellite cells (a type of stem cell), which can divide and differentiate into myofibers, and are critical components of muscle regeneration. The steps in muscle regeneration are fairly straightforward:
- Segmental injury or necrosis
- Invasion of the sarcoplasm by circulating monocytes, which differentiate into macrophages.
- Macrophages phagocytose cellular debris, “cleaning” up the sarcoplasm.
- Satellite cells enter the sarcoplasm and migrate towards the center of the of the myofiber.
- Satellite cells divide and form a tube (“myotube”) which produces sarcoplasm.
- The myotube extends to the edges of the damaged myofiber.
- The myotube expands, though is still narrower than the unaffected myofiber.
- A row of nuclei appear in the center of the regenerating myofiber, and sarcomeres begin to form.
Note that if the basal lamina has been destroyed, or if the damage affects a large area, then regeneration does not occur, and instead the muscle repairs itself via fibrosis (scarring).
Segmental necrosis and regeneration occur following a variety of insults, most commonly metabolic, nutritional (e.g. Selenium and vitamin E deficiency), or toxic (e.g. Ionophore toxicity)). Determining the etiology of the damage can therefore be quite difficult. It is helpful to observe the temporal pattern of the damage: are all muscle fibers at the same stage of necrosis or regeneration, suggesting a single, massive insult? Or is there a range of changes, with some fibers showing early stages of necrosis, and others at the end of regeneration, which suggests an on-going injury? These temporal changes are known as monophasic (occuring at one point) or polyphasic (an on-going proceess). These can be further classified by the commonly used distribution modifiers: focal, locally extensive, multifocal, or diffuse, to help narrrow down the etiology. For example, a focal, monophasic injury is more likely to be traumatic in origin than metabolic, while a multifocal, polyphasic injury could be due to the on-going lack of a nutritional requirement, as seen in Selenium and vitamin E deficiency.
1.4 Gross evaluation of muscle
Although important, the gross examination of muscles during a necropsy can be underwhelming, and if muscular disease is suspected, samples of muscle should always be submitted for histopathology, regardless of appearance. During a gross examination of a carcass, muscles should be evaluated for changes in size, texture, and colour. Muscles can be bigger (hypertrophied) or, more commonly, smaller (atrophied), or normal. Difficulty in assessing the normal size of muscles for different species and breeds can be aided by comparing with normal animals, or, if unilateral disease is present, with the contralateral side.
Changes in the colour of muscle are common, are often artifactual, and are dependent on blood perfusion, age, and species. Possible colour changes, along with potential causes, are listed below.
- Pale muscles:
- Normal in young animals
- Common in anemic animals
- Can be due to necrosis (ischemic)
- Denervation
- If streaking observed, usually due to necrosis and mineralization (e.g. Figure 1.3).
- Dark red:
- Congestion or hemorrhage
- Hemorrhagic necrosis
- Inflammation
- Hypostatic congestion (i.e. blood pooling due to gravity, a common artifact found during postmortem examinations)
- Green:
- Putrefaction (rot)
- Eosinophilic inflammation (rare – see Sarcocystosis)
The texture of diseased muscle can range from soft to hard (mineralized). Soft muscle can be indicative of necrosis, or potentially fat infiltration.
1.5 Biopsy techniques
As noted above, a biopsy of muscle should always accompany any suspected muscular disease. Muscle is highly susceptible to artifact, but proper preparation of the sample can help in ensuring a diagnostic sample. Muscle retains the ability to contract after biopsy/death, and this contraction can result in artifactual histological changes that mimic true pathological changes. These changes, noted as contraction band artifact, can be prevented relatively easily: shortly after removal, secure the muscle to a rigid surface prior to fixation. A simple and manageable technique whether in the field or clinic is to place a strip of muscle along a tongue depressor, and anchor it into place using two needles, staples, or sutures, prior to placing the sample in formalin. As a general rule, a muscle biopsy should not exceed ~ 1 cm in diameter (with myofibers running lengthwise).
Note: if submitting a sample of muscle with sharps in the container, make sure to notify the pathologist on the submission form.
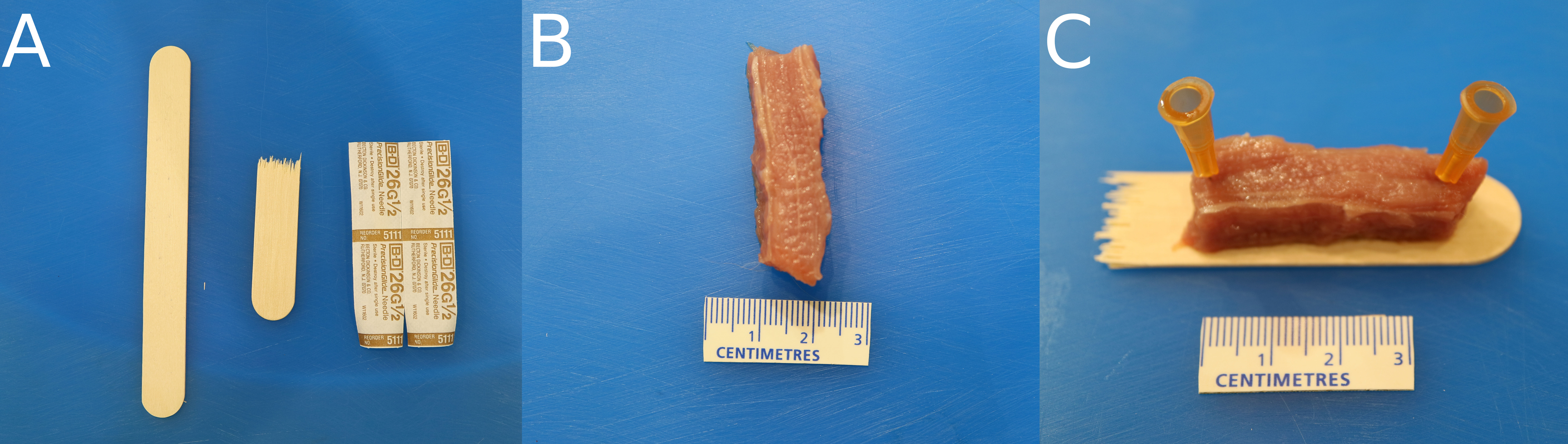
Figure 1.4: Appropriate technique for submitting muscle biopsies. A. Supplies that are readily available in most veterinary practices: a tongue depressor, broken to the appropriate size, and two small gauge needles. B. The diameter of a muscle biopsy should not exceed approximately 1 cm. C. Affix the biopsy to the tongue depressor using the two needles, staples, or sutures. Only a small amount of force is required to secure the muscle to the wood.